|
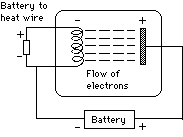
Now connect a second battery to the end of the coil, so that a current flows through the coil and heats it up. As the wire begins to glow, a current begins to flow, because now negatively charged particles are emitted from the hot wire, are attracted to the positive charge on the plate and by doing so, complete the electrical circuit.
Suppose the connections of the first battery are reversed, so that now the coil is positive and the plate is negative. Then no current flows, showing that the hot wire releases only negative particles, not positive ones. These particles were named electrons
In laboratory experiments these particles were directed by electrically charged structures (similar to the "electron guns" inside TV picture tubes) to form beams. Those beams were then bent by magnets and by electrified plates, and their behavior was studied. From such experiments and others the mass of the emitted particles, which became known as "electrons", could be determined. It turned out that they were rather lightweight. The simplest atom, that of hydrogen, contains a central positive particle, a proton, and a single electron, and the proton is nearly 2000 times heavier.
Light, like heat, can also knock electrons out of a metal. If the heated coil in the drawing is replaced by a clean metal plate, and light shines onto it, electrons are again released, and current will flow in the circuit. The explanation of this phenomena, called the photoelectric effect, earned Albert Einstein the 1921 Nobel Prize.
The same process will charge a spacecraft orbiting in the sunlight positively, to a few volts. Sunlight knocks out electrons from the surface and a few manage to escape, leaving the spacecraft positively charged; the situation then stabilizes, because the positive charge prevents any more electrons from leaving.
|
 Official GSFC Home Page
Official GSFC Home Page  NASA WWW Home Page
NASA WWW Home Page