#13. Energetic Particles
(Files in red–history)
 Index Index
 10a. Particle Drift
10a. Particle Drift
 11. Explorers 1/3
11. Explorers 1/3
 11a. Geiger Counter
11a. Geiger Counter
 12. Rad. Belts
12. Rad. Belts
 12H. Argus 1958
12H. Argus 1958
 12a. Inner Belt
12a. Inner Belt
 12b. Outer Belt
12b. Outer Belt
 13. Fast Particles
13. Fast Particles
 14. Synch. Orbit
14. Synch. Orbit
 15. Energy
15. Energy
 16. The Sun
16. The Sun
 16H. Schwabe, 1843
16H. Schwabe, 1843
 16a. Schwabe paper
16a. Schwabe paper
 16b. Carrington, 1859
16b. Carrington, 1859
 17. The Corona
17. The Corona
|
The atoms and molecules of a gas are in constant motion, colliding rapidly and filling all available space. The hotter the gas, the faster they move, and the more energy each of them holds. The free ions and electrons in a plasma behave the same way.
Knowing the temperature of the high atmosphere of Earth, or that of the Sun, we can calculate energies expected of ions and electrons found there. However, ions and electrons actually observed in space are often much, much more energetic, and may move at a respectable fraction of the velocity of light (300,000 km/sec or 186,000 miles/sec). One guesses that they get energized by electric and magnetic processes, not just by mere heat.
The Electron Volt
There is a convenient unit to measure such energies, the electron volt (ev). It is the energy gained by an electron (or proton, same size of electric charge) moving through a voltage difference of one volt.
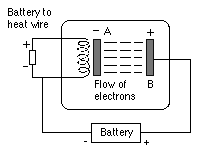
As described in the section on the electron, suppose that in a vacuum electrons come out of a hot slab A (drawing below), heated by a separate wire so that the voltage of the heating coil is not part of the circuit. Then if these electrons are attracted to a second plate B with a voltage (relative to A ) of +1 volt, each electron gains one electron volt. If the voltage is 10 volts, it gains ten times as much or 10 ev, a bit like a stone dropped from a height 10 times larger.
|
In the cathode-ray tube of a color TV (gradually being now replaced by flat screen, based on different principles), electrons are accelerated by about 30,000 volts, so that their energy when they hit the screen is about 30,000 ev. That is actually quite a lot: those electrons move at about 1/3 the velocity of light. But then, a TV picture tube is a quite sophisticated instrument. In a doctor's x-ray machine electrons are accelerated to energies 2-3 times higher, after which they hit a target and produce a spray of x-rays.
Particle Energies in Nature
How does nature compare?
- 0.03 ev
- The energy of a molecule of oxygen or nitrogen in the air we breathe. It moves as fast as a speeding bullet, but is still rather low on the scale of energies.
- 0.5 eV
- An atom or molecule at the temperature of the Sun's surface.
- 0.67 ev
- The energy needed by a proton or neutron to escape the Earth's gravity.
- 1000 - 15,000 ev
- Typical energy of an electron in the polar aurora.
- 40,000 ev
- Energy required by an electron to penetrate a thin-wall Geiger counter like that of Explorer 1.
- 50,000 ev
- Typical energy of an ion in the ring current.
Hold it!
We need bigger units:
- 1,000 ev = 1 kev (kilo-electron-volt, pronounced kay-ee-vee)
- 1,000,000 ev = 1 Mev (mega-electron volt or em-ee-vee)
- 1,000,000,000 ev = 1 Gev (giga-electron-volt or gee-ee-vee)
- 1.4 Mev
- The energy of electrons from radioactive potassium, a major source of the Earth's internal heat.
- 4.2 Mev
- The energy of alpha particles from radioactive uranium 238, another source of the Earth's heat (and of its helium as well--see positive ions, history).
- 10-100 Mev
- Typical proton energies in the inner radiation belt.
- 10-15,000 Mev
- Range of energies in solar outbursts (see Sun).
- 1-100,000,000,000 Gev
- Range of energies among cosmic ray ions. However as their energy goes up, their intensity goes way down, so that ions at the high energy end are quite rare.
Note on Relativity
While the theory of relativity allows no particle with mass to move with a velocity exceeding (or even equaling) that of light, there is no limit on its energy. Close to the speed of light, however, the addition of energy only slightly increases the velocity. An ion accelerating from 0.9 to 0.99 times the speed of light needs several times more energy than the amount it needed to reach 0.9 times in the first place, though its energy makes it considerably heavier.
Why and How
Where do single electrons and ions acquire such high energies? Excellent question. We guess magnetic and electric fields may be involved, and have learned a great deal in that direction, but the exact processes (probably more than one) remain to be nailed down. Acceleration takes place in solar flares and CMEs (see Sun) but, like a clever conjuring trick, although it happens right in front of our eyes, we still don't get it.
Powerful shocks--abrupt discontinuities piled up in front of rapidly moving gas--can also do it, and at least one interesting event of this sort was observed in the Earth's magnetosphere. The most powerful shocks occur in the envelope of gas expanding from the site of supernovas, and it is widely believed that such shocks (which carry a great amount of energy) are the source of most cosmic ray particles.
All those are good reasons to study the acceleration of particles in the aurora and radiation belts. The energies are more moderate, but the processes occur in a region of space which instrumented satellites can probe. As we study such acceleration processes we gradually learn how plasmas and magnetic fields interact in space, and that experience can then be applied to the rest of the universe.
|
