#7. Plasma
(Files in red–history)
 Index Index
 4. Electrons
4. Electrons
 4H. Thomson, 1896
4H. Thomson, 1896
 4a. Electric Fluid
4a. Electric Fluid
 5. Field Lines
5. Field Lines
 5H. Faraday 1846
5H. Faraday 1846
 6. EM Waves
6. EM Waves
 7. Plasma
7. Plasma
 7a. Fluorescent lamp
7a. Fluorescent lamp
 7H. Langmuir, 1927
7H. Langmuir, 1927
 8. Positive Ions
8. Positive Ions
 8H. Arrhenius, 1884
8H. Arrhenius, 1884
 9. Magnetic trapping
9. Magnetic trapping
|
Plasma is sometimes called "the fourth state of matter", beyond the familiar three--solid, liquid and gas. It is a gas in which atoms have been broken up into free-floating negative electrons and positive ions, atoms which have lost electrons and are left with a positive electric charge.
In the lower atmosphere where we live, any atom that loses an electron (say, by being hit by a fast cosmic ray particle) soon recaptures it or one like it. The situation is quite different at high temperatures, such as exist on the Sun. The hotter the gas, the faster its atoms and molecules move, and at very high temperatures, the collisions between such fast-moving atoms are violent enough to rip off electrons. In the Sun's atmosphere, a large fraction of the atoms at any time is "ionized" by such collisions, and the gas acts as a plasma.
Unlike cool gases (e.g. air at room temperature), plasmas conduct electricity and are strongly affected by magnetic fields. The fluorescent lamp, widely used in the home and at work, contains a rarefied inert gas with a fraction of a percent mercury vapor, which produces a plasma when heated and agitated by electricity, from the power line to which the lamp is connected. The power line makes one end electrically positive, the other negative (see drawing below) causing (+) ions to be accelerated towards the (-) end, and (-) electrons to the (+) end. The accelerated particles gain energy, collide with atoms, eject additional electrons and thus maintain the plasma, even if some other particles re-combine. The collisions also cause mercury atoms to emit light, and in fact, this source of light is more efficient than conventional lightbulbs. Neon signs and streetlights operate on a similar principle, and some plasma devices are (or were) used in electronics.
|

[In case you ask: when the fluorescent lamp is first turned on, the gas is cold, but a few free ions and electrons are always present, due to cosmic rays and natural radioactivity. The filaments at the ends also release electrons. Collisions quickly multiply their number.
And it is true that since alternating current is used, the location of (+) and (-) in the above drawing switches back and forth 60 times each second. However, ions and electrons respond much faster than that, hence the process stays the same. Click here for more about the fluorescent lamp]
As noted, the Sun consists of plasma. Another important plasma in nature is the ionosphere, starting about 70-80 km above ground. Here electrons are torn off atoms by sunlight of short wavelengths, ranging from the ultra-violet to X-rays: they do not recombine too readily because the atmosphere becomes increasingly rarefied at high altitudes and collisions are not frequent. The lowest part of the ionosphere, the "D layer" at 70-90 km, still has enough collisions to cause it to disappear after sunset. Then the remaining ions and electrons recombine, while in the absence of sunlight new ones are no longer produced. However, that layer is re-established at sunrise. Above 200 km, collisions are so infrequent that the ionosphere persists day and night.
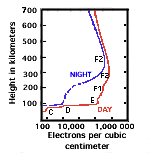
The topside ionosphere extends many thousands of km into space and merges with the magnetosphere, whose plasmas are generally more rarefied but also much hotter. The ions and electrons of the magnetospheric plasma come in part from the ionosphere below, in part from the solar wind (next paragraph), and many details of their entry and heating are still unclear.
Finally, there exists the interplanetary plasma--the solar wind. The outermost layer of the Sun, the corona, is so hot that not only are all its atoms ionized, but those which have started off with many electrons have several of them (sometimes all of them) torn off, including deeper-lying electrons which are more strongly attached. For instance, characteristic light has been detected in the corona from iron which has lost 13 electrons.
This extreme temperature also prevents the plasma of the corona from being held captive by the Sun's gravity, and instead it flows out in all directions, filling the solar system far beyond the most distant known planets. Through the solar wind the Sun shapes the Earth's distant magnetic field, and the wind's fast flow (~400 km/s) supplies the energy which ultimately powers the polar aurora, the radiation belts and magnetic storm phenomena.
Further Reading:
Plasma physics is a difficult, mathematical field, whose study requires a thorough understanding of electromagnetic theory. Some college texts on electricity and magnetism deal with aspects of plasma physics, e.g. chapter 10 of "Classical Electrodynamics" by J.D. Jackson.
Questions from Users:
*** Electric and Magnetic Energy
*** How does one contain a Plasma?
***
Can plasma physics explain ball lightning?
***
Is fire a plasma?
|